Screening Selected Maize Single Crosses for Tolerance to Low P in Acidic Soils of Bumala and Maseno
1Olung’ati O.E, Kiplagat O, 2Gudu .S.O, Ouma E, 3Ochuodgo J.
1Department of Biotechnology, University of Eldoret
2Department of Agronomy and Animal Science, Rongo University
3Department of Seed Crop and Horticultural Sciences, University of Eldoret
Abstract
Generally, 13% of Kenya’s arable land mass (7.5 million ha) is acidic and prone to poor phosphorus (P) availability and soil acidity. This results in crop yield losses due to the direct adverse effects Aluminium toxicity and P deficiency due to fixation of this element in the soil. The objective of this study was to develop and select P efficient maize single crosses developed by crossing as per North Carolina II mating design. Sixty maize genotypes, among them 34 single crosses were screened under acidic soils in Bumala and Maseno in a randomised complete block design. Sixty-seven percent of these single crosses were efficient, while 33% were inefficient. Two percent were efficient and responsive, 14% were inefficient but responsive, and the 79% were efficient but non-responsive. Generally, GY had a positive correlation with EH (0.45) and PH (0.61), while PH and EH had a positive correlation (r=0.86) for the single crosses. The addition of P had significant effect on the grain yield, plant height, ear height and flowering of the genotypes at Bumala and Maseno. However the effect of 26kgP/ha was marginal at Maseno as compared to Bumala. The sites and genotypes varied significantly with regard to soil analysis and grain yield respectively, with the efficient and responsive genotypes selected for use in low input farming systems. Also, some of the efficient but non-responsive lines can also be selected for low input farming.
Keywords: Maize, Single crosses, Aluminium toxicity, P efficiency
Introduction
Globally, maize is considered as the third in importance after wheat and rice among consumed cereals (Ali et al., 2013; Onasanya, 2009). In Sub Saharan Africa and Kenya, the cereal is ascribed a unique importance because of its value as a staple food crop, an industrial raw material and an animal feed (Magenya et al., 2008). Produced by both small and large scale farmers, maize provides Kenyans with a source for approximately 35.7% of consumed calories (Gichuru, 2013) due to its staple status. However, its production is hampered by both biotic and abiotic constraints (pests, diseases, poor weather and soil conditions, poor seed quality, etc.). Soils that are categorised as being low in available P are however considered major challenge to maize productivity (Ouma et al., 2015). Also according to Magenya et al (2008) and Gichuru (2013), P sorption in acid soils that leads to P deficiency, poor soil fertility, significantly lowers maize productivity. In Kenya, approximately 7.5 million ha of maize producing as well as agriculturally viable land is acidic (Kisinyo et al., 2014). In such soils, applied P becomes fixed due to acidity and concurrent aluminium toxicity (Mumtaz et al., 2014), thus making chemical amelioration less effective. In western Kenya, soil acidity is a common occurrence and is commonly associated with aluminium toxicity as well as P deficiency. Such areas are reported as expressing an available soil P of between 2-5 mg/kg, whereas the minimum threshold for maize production is set at 10mg/kg (Brink and Bellay, 2006; Yang et al., 2013; Kisinyo et al., 2014; Ouma et al., 2012). This situation has resulted in yield losses of between 20%-58%, while its accompanying Al toxicity has resulted in yield losses of between 16%-45% (Kisinyo et al, 2011; Ouma et al.,2012). Addressing the issue of low P availability therefore becomes vital in Western Kenya.
Phosphorus is among the major crop nutrients required for growth and development of crops, and deficiencies of this mineral leads to negative effects on the crop’s development process and eventually reducing yield (Ward et al., 2011). As a major nutrient, this element is required in significantly larger amounts, similar to Nitrogen and Potassium. It is an important molecule in the ATP molecular structure as well as in phospholipids and nucleic acids and is an essential requirement in photosynthesis (Obura et al., 2009). In addition to these roles, Phosphorus availability is important in minimizing Aluminium induced root damage, and ameliorating accumulation of the toxin in root tips cells (Sun et al.,2008). In terms of importance, the fully oxidised and inorganic form of this mineral element is considered as the most important form for plant use due to its function in the above mentioned roles (Satyaprakash et al., 2017). In addition to soil acidity and Al toxicity, P availability is also influenced by lack of or presence of organic matter in soil as well as the continual use of acidic fertilisers (Sharma et al.,2013; Ware, 2006). Crops exposed to low P conditions express symptoms such as the progressive purpling of leaves from tips to the margins and eventually the whole leaf, necrosis of the stem, poor flowering and seed fill, poor yields, and the complete elimination of young susceptible plants (Fageria, 2009).
Traditionally, P deficiency can be ameliorated by application of lime or organic and inorganic fertiliser’s (Kisinyo, 2011). However, in most P deficient soils, only 20% of applied P remains available for plant use and acquisition in a majority of soil ecosystems due to fixation of the remaining 80% (Balemi & Ngeshio 2012; Mumtaz et al., 2008). This then, due to the diverse nature of soil, leads to the development of P depleted pockets supplying between 10-15% of supplied P (Obura et al., 2014), and a process that may eventually lack economic sustainability. In addition, liming or the use of other soil amendments, is considerably expensive for small scale farmers (Kisinyo et al., 2014; Ouma et al., 2012), and it is therefore prudent to explore genetically conditioned tolerance. According to Kisinyo et al(2014), maize genotypes with adequate phosphorus utilization potential (PUE), or those adopted to enhanced acquisition of P (PAE) become and indispensable tool in dealing with the constrain of low available P especially for low income small scale farmers. This study therefore aimed at selecting P efficient F1s from an F1 group developed by crossing.
Materials and Methodology
Study Sites
The study was carried out in three different locations, Rongo University, Maseno University and Bumala. Rongo University farm was the crossing site for development of single cross hybrids, Maseno university farm and the farmer’s fields in Bumala were the sites for evaluation of the F1 hybrids. Rongo University is located at between 1300-1500m a.s.l, receives an average of 1600mm of rainfall pa, and experiences a temperature of between 20-21.7ᵒC (Low, 1997; MoA, 2014). Bumala is located between 1135-1500m a.s.l, experiences a temperature of between 20.5-22.7ᵒC, receives an annual rainfall of approximately 900-1700mm, and is reported as having acidic soil with ah pH of 4.5-4.6 and available soil P of 2.74 mg/kg (Ouma et al.,2012). Maseno is located approximately 1500m a.s.l, experiencing a rainfall average of 1750mm pa, a temperature of 28.7ᵒC, and is has acidic soil of pH 4.5-5.4 with an available soil P of 4.5 mg/kg (Gichimu et al.,2009). While soil s in Bumala are classified as orthic ferralsols (Ouma et al., 2012), Maseno soils are classified as dystric nitisols (Mwai et al., 2001).
Genetic material
The germplasm used in the study was sourced from Rongo University and the University of Kwazulu Natal. From Rongo university was sourced the 14 Aluminium tolerant and P efficient maize genotypes, while the 9 Maize streak virus tolerant genotypes were sourced from the University of Kwazulu Natal. The material from the University of Kwazulu Natal were all inbred lines, while from Rongo University ware a mixture of inbred lines, and populations from Brazilian introductions (Ouma et al., 2012).
Development of Single Cross hybrids
Crossing was done in Rongo, at the University collage field in the short rains of 2015, using the North Carolina II mating design with the Kenyan inbreds as female and South African inbreds as male. Pollen was transferred in pollen bags set up overnight from the tassel of the male plants to the silks of the female pants at 9 am in the morning to 10 am during the two week flowering period. It resulted in 34 single cross genotypes for screening. The crossing block consisted of two plots staggered by a week and a half, each consisting of two plots (male and female), with each male and female having two rows for each of the genotypes.
Field screening for tolerance to Maize streak virus, Aluminium toxic/ P efficient. The field experiment was laid out in RCBD with the genotypes and two P levels, P0 and P1, as treatments. Each treatment was replicated twice. A total of 34 single crosses, 2 repeats, 23 parietals and 1 check were evaluated. Each plot had ten genotypes and each block has six plots, with each genotype represented in singular rows (3m long, with 0.75 m inter-row spacing and 0.25 m intra-row spacing) (Gichuru, 2013; Ngwira & Khonje, 2005; Scott et al., 2009).
The genotypes under screening were subjected to two rates of P , (P0) 0kgP/ha and (P1) 26Kg P/Ha) while N was applied at 30kg N/Ha at planting and at knee height 45Kg N/ Ha (NAFIS; Gudu et al., 2011; Gudu et al., 2005). Due to its capacity to supply N at 18%, which is equivalent to 6.48 kg of N per 100kg DAP, the treatment with –P (P0) was supplied slightly more CAN than +P (26kgP/ha) to balance the 18% N supplied by DAP.
P-deficiency tolerance assessment
P efficiency was evaluated on basis of grain yield (GY), plant height (PH), ear height (EH), days to 50% silking and 50% anthesis. Data was collected for all these traits for P efficiency assessment (Jiang et al, 2010; Too et al, 2014), with plant height and ear height being collected at plant maturity.
Model: Yijk=µ+ bi+Pj+Gk+PGjk+Ɛijk
Where; µ-general mean, b- blocking effect, G- Genotype effect, P- Treatment effect, PG- treatment genotype interaction, Ɛ-error term
% Response to P application of P for the various inbred lines and single cross hybrids was calculated according to Ouma et al (2012) as:
Data analysis
The data for analysis was subjected to ANOVA in GENSTAT version 14 and mean separation done using DMRT test at 5% level of significance.
Soil Sampling
Soil sampling was done using systemic quadrates (Midwest Laboratories, 2004; Okalebo et al., 2002), and the zigzag method (Okalebo et al., 2002). The method used nested the zigzag systems within the quadrates. The sub-samples were mixed thoroughly and approximately 1.2 kg composite samples were packed in a black polythene bags and transported to the laboratory for were air-drying, grinding and sieving via a 2 mm sieve. The samples were then tested for pH using the HANNA soil analysis tool kit (Vanek, 2017), texture and organic carbon according to Okalebo et al (2002), and available P using Olsen et al (1954).
Results
Soil testing results
Results of the soil analysis showed that the two sites were generally low in fertility, and also had low pH and available P. Bumala had a pH 4.6, while Maseno had a pH was 5.2. These values indicated that the two sites have strongly acidic soils (Table 1), with Maseno having a slightly higher available P. The two sites also had low C level but Maseno had a slight advantage over Bumala for this trait as well.
Table 1: Soil analysis results for the experimental plots used in the study at Maseno and Bumala.
|
SITE |
Organic Carbon |
P |
pH |
Textural Class |
Soil Type |
|
|
BUMALA |
2.66 |
3.3 |
4.6 |
Sandy clay loam |
Orthic ferasols |
|
|
MASENO |
3.18 |
4.8 |
5.2 |
Clay loam |
Dystric Nitrisols |
|
Response in P deficient/ low P conditions in Acidic soils of Maseno and Bumala
The genotypes used in the study varied significantly in terms of PH, EH, GY, days to 50% silking and days to 50% tasseling (p<0.05), with the single cross hybrids outperforming the parental inbred lines under both low P (0kgP/ha)and high P (26kgP/ha) conditions. The interaction between the genotypes and sites was significant (p <0.05) for GY, PH, EH, days to 50% tasselling and days to 50% silking. The interaction between sites and P was significant (p <0.05) for GY, PH, EH, and days to 50% tasselling but not for days to 50% silking, while the interaction between genotypes and P was significant (p <0.05) for GY, PH, EH. Only PH and EH were significantly affected by the interaction between site, genotype and P (Table 2).
Table 2: Mean square tale for maize genotypes tested for P efficiency under field condition in Bumala and Maseno
|
Source of variation |
d.f. |
GY |
PH |
EH |
SILK |
TASS |
|
SITE |
1 |
109.712*** |
911121*** |
273537.4*** |
3.98 |
150.1** |
|
GENOTYPE |
59 |
5.5897*** |
305881*** |
24345.9*** |
244.31*** |
196.08*** |
|
P |
1 |
16.3042*** |
157049*** |
85166.5*** |
1520.22*** |
1535.74*** |
|
SITE.GENOTYPE |
59 |
1.7929*** |
326699*** |
3643.7*** |
65.19*** |
52.36*** |
|
SITE.P |
1 |
4.3532*** |
12386*** |
182261.1*** |
49.31 |
186.74** |
|
GENOTYPE.P |
59 |
1.0631*** |
5337*** |
2142.7*** |
30.77 |
32.62 |
|
SITE.GENOTYPE.P |
59 |
0.5285 |
9449*** |
3679.4*** |
37.05 |
23.42 |
|
Residual |
239 |
0.3874 |
1218 |
475.3 |
5.7 |
27.46 |
|
Total |
478 |
|||||
|
MEAN |
2.119 |
148.03 |
50.07 |
73.54 |
68.42 |
|
|
SE |
0.6224 |
34.904 |
21.801 |
5.694 |
5.241 |
|
|
CV |
29.4 |
4.2 |
13.5 |
7.7 |
7.7 |
Note *, ** and *** indicates significance at P ≤ 0.05, P ≤0.01, P ≤ 0.001 levels, respectively.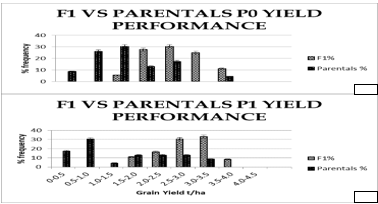
With regard to the performance of the single crosses across the sites in P0 5.6% expressed a GY of 1.0-1.49 t/ha, 27.8% expressed a GY of 1.5-2.0t/ha, 30.6% expressed a GY of 2.0-2.49t/ha, 25% expressed a GY of 2.5-3.0t/ha, 11.1% expressed a GY of 3.0-3.49t/ha. This performance was better than the parental lines that had 8.7% expressing 0-0.49 t/ha, 26.1% expressing 0.5-0.99 t/ha, 30.4% expressing 1.0-1.49 t/ha, 13.0% expressed a GY of 1.5-2.0 t/ha, 17.4% expressed a GY of 2.0-2.49t/ha, 0% a GY of 2.5-3.0t/ha, and 4.3% expressed a GY of 3.0-3.49t/ha. On the other hand, all the single cross hybrids expressed a GY of greater than 1t/ha under 26kg/ha P, while only 47.82% of the parental inbred lines expressed the same grain yield under 26kg/ha P, and majority of the parental inbred lines (52.18%) expressed a grain yield of below 1t/ha under 26kg/ha P.
The best single cross in Maseno under low P (P0) was 4BXSYNAL with a GY of 5.4 t/ha, and the worst was 13MAK-13BXKML036 with a GY of 0.94 t/ha. Under high P (P1), the best single cross in Maseno was 4BXSYNAL with a GY of 6.23t/ha, and the worst was 13MAK-13BXKML036 with a GY of 1.90 t/ha. The best single cross in Bumala under low P was 203B-9X54B with a GY of 1.2 t/ha, while the worst was 203B-9X4B with a GY of 0.09 t/ha. Under high P the best single cross in Bumala was 1BXBRC with a GY of 2.1 t/ha and the worst was 9BXCON5 with a GY of 0.46 t/ha. Across the two sites, the best single cross under low P was 4BXSYNAL with a GY of 3.3 t/ha, while the worst single cross was 13MAK-13BXKML036 with a grain yield of 1.10. Under high P, the best single cross across the sites was 4BXSYNAL with a GY of 4.42 t/ha and the worst was 13MAK-13BXKML036 with a grain yield of 1.75 t/ha (Table 6). Comparatively, addition of P had a 21.1% incremental effect on the grain yield of the single cross F1 used in the study, with a 7.1% increase of grain yield in Maseno and a 29.1% increase of grain yield in Bumala due to addition of P. Also, the overall grain yield for the F1 hybrids in Maseno was 38.5% higher than in Bumala. In addition, the addition of P had a significant effect in improving ear height (EH) and plant height (PH), as well as reducing the days to 50% tasseling, and reducing the days to 50% silking. The single cross hybrids were further classified into different categories (tolerance to low P soils, and responsiveness to P) based on their grain yield at the different P levels. The classification resulted in the conclusion that 97.1% were efficient, 17.65% were responsive to P, and 14.71% were both P efficient and responsive (Table 6).
Table 3: Means for traits under low P and High P in acidic soils of Maseno and Bumala. Means across the same row with different letters differed significantly
|
Trait Measured |
P0 |
P1 |
MEAN |
SED |
|
Grain yield |
0.421a |
1.054b |
0.737 |
0.369 |
|
Plant height |
114.12a |
151.6b |
132.86 |
13.817 |
|
Ear height |
28.31a |
51.72b |
40.01 |
8.118 |
|
Days to 50% tasseling |
69.82a |
65.14b |
67.48 |
3.98 |
|
Days to 50% silking |
75.56a |
71.31a |
73.43 |
4.644 |
Table 4: Means for traits across sites (Maseno and Bumala). Means across the same row with different letters differed significantly
|
|
Maseno |
Bumala |
MEAN |
SED |
|
GY |
2.597a |
1.641b |
2.119 |
0.6224 |
|
PH |
165.81a |
130.24b |
148.03 |
34.904 |
|
EH |
59.82a |
40.32b |
50.07 |
21.801 |
|
Days to 50% tasseling |
68.98a |
67.86a |
68.42 |
3.98 |
|
Days to 50% silking |
73.45a |
73.63a |
73.54 |
4.644 |
Phenotypic relationships among maize performance indicator traits across sites
GY had a strong and positive correlation with both EH (r=0.82) and PH (r=0.80) for the all genotypes in the study. Also, PH and EH had strong positive correlation (r=0.82) for all the genotypes in the study. For the inbred lines, correlation between GY and EH, GY and PH, and EH and PH was strong and positive at (r=0.87), (r=0.93), and (r=0.96) respectively. For the single cross hybrids, correlation between PH and EH was positive and strong (r=0.86), correlation between PH and GY was moderate and positive (0.61), while correlation between EH and GY was weak and positive (0.45). For all the genotypes in the study, GY had a positive and insignificant correlation with days to 50% tasseling (r=0.01) and with days to 50% silking (r=0.09), this was similar to the F1, where GY had a positive yet insignificant correlation with days to 50% tasseling (r=-0.06) and with days to 50% silking (0.17). For the inbred lines correlation between GY and days to 50% tasseling (r=0.41), and GY and days to 50%silking (r=0.50) was moderate and positive (Table 4).
Table 5: Correlation between GY, EH, PH and days to 50% flowering of all genotypes tested for P-efficiency in Maseno and Bumala.
|
|
Grain Yield |
Plant height |
Ear Height |
Days to 50% Tasseling |
Days to 50% Silking |
|
Grain Yield |
1 |
||||
|
Plant height |
0.8** |
1 |
|||
|
Ear Height |
0.82** |
0.82** |
1 |
||
|
Days to 50% Tasseling |
0.01 |
0.15 |
0.25* |
1 |
|
|
Days to 50% Silking |
0.09 |
0.3 |
0.39* |
0.92** |
1 |
* and **; Significance at 5 and 1%, respectively.
Table 5: Table with Selected F1s for grouped for P efficiency and response to P based on Grain yield means.
|
Grain Yield |
Response to P |
|||
|
GENOTYPE |
P0 |
P1 |
P1/P0 (R) |
Categories |
|
4BXSYNAL |
3.34 |
4.42 |
1.33 |
EN |
|
54BXBRC |
3.20 |
2.88 |
0.90 |
EN |
|
9BXSYNAL |
3.18 |
3.44 |
1.08 |
EN |
|
203B-9X54B |
3.14 |
3.01 |
0.96 |
EN |
|
44BX203B-9 |
2.89 |
3.06 |
1.06 |
EN |
|
44BX203B-14 |
2.85 |
2.73 |
0.96 |
EN |
|
41BX203B |
2.69 |
2.36 |
0.88 |
EN |
|
4BX203B-1 |
2.65 |
2.28 |
0.86 |
EN |
|
41BXCON5 |
2.31 |
2.74 |
1.19 |
EN |
|
13BX203B-14 |
2.17 |
3.67 |
1.69 |
ER |
|
203B-9X4B |
2.13 |
3.24 |
1.52 |
ER |
|
41BXBRC |
1.59 |
3.76 |
2.36 |
IR |
|
1BXBRC |
1.57 |
3.54 |
2.25 |
IR |
|
1BXAO809 |
1.53 |
1.96 |
1.28 |
IN |
|
13BXKML036 |
1.10 |
1.75 |
1.59 |
IR |
KEY: I, Inefficient; R, Responsive, E, efficient; N, non-responsive; IR, inefficient and responsive; ER, efficient and responsive; EN, efficient and nonresponsive; IN, inefficient and nonresponsive.
Discussion
Variation in agronomic traits due to P addition, genotypic differences, and site
The genotypes and P treatments had a significant effect on the plant height, ear height, grain yield, and days to flowering of the genotypes in the study. At Bumala the addition of P led to an overall increase in grain yield by 29.1%, plant height by 24.7% and ear height by 45.8% but reduced days to 50% tasseling by 7.2% and days to 50% silking by 5.6%. In Maseno, grain yield was increased by 7.1%, plant height by 0.42% and ear height by 8.8%, days to 50% tasseling and days to 50% silking reduced by 3.4% and 4.1% respectively due to the addition of P. This effect of P in improving agronomic crop traits identified in this study relates well with other studies. Fosu-Mensah & Mensah (2016), Ouma et al (2013),Umeri et al (2016) reported that the addition of P resulted in a reduction of days to flowering (tasseling and silking), as well as an increase in grain yield. According to Ouma et al (2013), P addition at 26kgP/ha resulted in a grain yield increase by a margin of 73.5%, as well as a corresponding increase in ear and plant height. Besides these findings, Lokhande et al (2015) concluded that an increase in wet and dry biomass, as well as increased plant height for Coriander due to application of P at 45kgP/ha. Also, Alias et al (2003) concluded a 15% increase in plant height and a 53.76% increase in grain yield due to P supply at 150kgP/ha for maize. On the other hand, Dai et al (2013) reported a yield loss of 560kg/ha/year for maize due to lack of P fertiliser thus indicating the mineral’s importance to improve grain yields for the crop.
In addition, the different genotypes expressed varied grain yield response due to P addition. This could have been due to inherent genetic differeces in acquiring P from the soil. These results are similar to those reported by Fosu-Mensah & Mensah (2016) as well as Ouma et al (2013) and Umeri et al (2016), who reported that genetic differences among genotypes contributed to variance in acquisition and utilization of soil P. Although the grain yield at Maseno was better than Bumala the effect of P addition was marginal at Maseno as compared to Bumala. This difference may be due to the higher soil P, variation in response to added P at 26kgP/ha at the two sites, and higher soil pH at Maseno as compared to Bumala. These results are comparable to those of (Kihara, 2016) who reported variation in the effect of NPK fertiliser on grain yield across sites. The soils in these two regions also expressed a variation in organic carbon content with Maseno having a slightly higher level. High organic carbon is known to increase the soil CEC which affects retention of cation nutrients, as well as improving water infiltration and retention (Noellemeyer & Six, 2015). In addition to the soil issues, the variation in rain fall received by these two regions may also have affected overall grain yield.
The reported improvements in plant grain yield and yield components due to the addition of P can be ascribed to its role in the development of plant roots as these are the main water and mineral absorption organs. In addition, P is essential in photosynthesis, cell division and elongation, and being synergistic to Nitrogen absorption (Ouma et al., 2013; Salehi & Anampanah, 2015). Despite the improved yields due to P addition, the overall yields of the genotypes in the study at Bumala neither met the 3t/ha threshold set by Gudu (2011), nor did they meet 3.41-8.7t/ha threshold set by Ouma et al (2012). These results can be attributed to the prolonged drought at Bumala during the experimental season that also interacted with late flowering for some of the genotypes. According to Halindu (2015), prolonged drought can cause yield losses of between 50-100% depending on the length of the scourge. At the study site in Bumala, nearby fields and the farmer’s field had visible effects of the drought with losses of up to 100% for some of the neighbouring farms.
Phenotypic relationships among maize performance indicator traits across sites
PH and EH had a positive and significant correlation to each other as well as to GY. These results are in agreement with those of Appiah et al (2014). Such positive correlation presents that these yield components are associated positively with GY and can be used effectively in selection purposes for GY (Akongwubel et al., 2012). Between grain yield and flowering the general correlation was positive but weak and insignificant for all the genotypes in the study and for the F1s. These findings are similar to those reported by Yousuf and Saleem (2001). However, the correlation between grain yield and flowering dates was positive and moderate for parental inbred lines, where GY and days to 50% tasseling (r=0.41), and GY and 50/5 silking (r=0.50).
Conclusion
The study identified 23 P efficient single crosses and 11 inefficient single crosses. Of the efficient single crosses, only two were responsive to P addition, while of the inefficient three were responsive to P addition. These genotypes can therefore be utilized in further development of three way and top crosses for P efficiency as well as gene pyramiding to develop multiple tolerant maize genotypes. Also, genotypic variability for P efficiency and response to P was identified among the maize genotypes in study and was further enhance by soil fertility variation among the sites.
References
Ali Q, Ahsan M., Ali F., Muhammad S., Manzoor M., Khan N.H.,Basra S.M.A., and Bin Mustafa H.S.(2013). Genetic advance, heritability, correlation, heterosis and heterobeltiosis for morphological traits of maize (Zea mays L). Albanian j. agric. sci. 2013;12 (4): 689-698, 689-698.
Alias, A., Usman, M., Ullah , E., & Warraich, E. A. (2003). Effects of Different Phosphorus Levels on the Growth and Yield of Two Cultivars of Maize (Zea mays L.). International Journal of Agriculture and Biology, 5(4), 632-634.
Akongwubel, A. O., Ewa, U. B., prince, A., Jude, O., Martins, A., Simon, O., & Nicholas O. (2012). Evaluation of Agronomic Performance of Maize (Zea mays L.) under Different Rates of Poultry Manure Application in an Ultisol of Obubra, Cross River State, Nigeria, International Journal of Agriculture and Forestry, 2(4), 138-144.
Appiah, A. K., Helget, R., Xu , Y., Mueller, N., & Wu, J. (2014). Response of Soybean Yield and Yield Components to Phosphorus Fertilization in South Dakota. Annual Conference on Applied Statistics in Agriculture (pp. 1-19). Kansas State : Kansas State University.
Balemi, T., & Negisho, K. (2012). Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: a review. Journal Of Soil Science And Plant Nutrition, 12(3), 547-562.
Barasa J.N. , Omami E.N., Okalebo J.R. & Othieno C.O. (2013). Effect of lime and phosphorus fertilizer applications on performance of french beans in Uasin Gishu district, Kenya. Global journal of biology, agriculture and health sciences, 35-41.
Brink M & Bellay G. (2006). Cereals and Pulses. Wagenigen: Backhuys Publishers.
Dai X, Ouyang Z, Li Y, Wang H (2013) Variation in Yield Gap Induced by Nitrogen, Phosphorus and Potassium Fertilizer in North China Plain. PLoS ONE 8(12): e82147. doi:10.1371/journal.pone.0082147
Fageria, N. (2009). The use of nutrients in crop plants. Boca Raton: CRC Press.
Fosu Mensah , B. Y., & Mensah, M. (2016). The effect of phosphorus and nitrogen fertilizers on grain yield, nutrient uptake and use efficiency of two maize (Zea mays L.) varieties under rain fed condition on Haplic Lixisol in the forest savannah transition zone of Ghana. Environmental Systems Research, 5(22) , 2-17.
Gichimu B. M, Owuor B.O, & Dida M.M. (2008). Agronomic Performance Of three most popular Commercial Watermelon Cultivar In Kenya As compared to One Newly Introduced Cultivar And One Local Landrace Grown on Dystric Nitrisols under Bub- humid Tropical Conditions. Journal of Agricultural and Biological Science, 3 (5&6), 65-71.
Gichuru, L. N. (2013). Breeding investigations on the utility of maize streak resistant germplasm for hybrid development in the tropics. Scotsville; South Africa: University of KwaZulu- Natal. PHD Thesis.
Gudu, S., Okalebo , J., Othieno, C., Obura, P., Ligeyo, D., Shulze , D., et al. (2005). Response of five maize genotypes to nitrogen, phosphorus and lime on acid soils of western Kenya. African Crop Science Conference Proceedings, Vol. 7. pp. 1109-1115.
Gudu S, Ligeyo D,Ouma E,Matonyei T, Onkware A.O, Othieno C.O, Okalebo J.R,Too E.J, Agalo J,Kisinyo P.O,Ochuodho J.O and Were B. (2011). Screening for tolerance to Al toxicity and P- efficiency in Kenyan maize germplasm. Eldoret: Moi University.
Halindu, J., Abubkar, L., Izge, U. A., Ado, G. S., Yakubu, H., & Haliru, S. (2015). Correlation Analysis for Maize Grain Yield, other Agronomic parameters and Sriga affected Traits Under Striga infested/ Free Environment. Journal Of Plant Breeding and Crop Science,7(1), 9-17.
Jiang , H., Yang , J., & Zhan, J. (2010). Screening of tolerant maize genotypes in the low phosphorus field soil. World Congress of Soil Science, Soil Solutions for a Changing World , (pp. 214-217). Brisbane, Australia.
Kihara J., Nziguheba G., Zingore S., Coulibaly A., Esilaba A., Kabambe V., Njoroge S., Palm C., Huising J. (2016). Understanding variability in crop response to fertilizer and amendments in sub-Saharan Africa, In Agriculture, Ecosystems & Environment, 229,1-12. https://doi.org/10.1016/j.agee.2016.05.012.
Kisinyo., P. (2011). Constraints of Soil Acidity and nutrient depletion on maize production in Kenya. Eldoret: Moi University; PhD. Thesis,.
Kisinyo, P., Opala, P., Gudu, S., Othieno, C., Okalebo, J., Palapala, V., & Otinga, A. (2014). Recent advances towards understanding and managing Kenyan acid soils for improved crop production. African Journal Of Agricultural Research, 9(31), 2397- 2408. http://dx.doi.org/10.5897/ajar2013.8359.
Lokhande, S. N., Jogdande, N. D., & Thakare , S. S. (2015). Effect of Varying levels of Nitrogen and Nhosphorus on Nrowth and Need Yield of Coriander (Coriandrum Sativum). Plant Archives,15( 1),57-59.
Low, J., Kinyae, P., Gichuki, S., Oyunga, M., Hagenimana, V., & Kabira, J. (1997). Combating vitamin A deficiency through the use of sweet potato. Lima, Peru: International Potato Center.
Mafu, N. F. (2013). Marker-Assisted Selection for Maize Streak Virus Resistance and Concomitant Conventional Selection for Downy Mildew Resistance in a Maize Population. Scotsville; South Africa: University of KwaZulu-Natal; Msc Thesis.
Magenya, O., Mueke , J., & Omwega, C. (2008 ). Significance and transmission of maize streak virus disease in Africa and options for management: A review African Journal of Biotechnology Vol. 7 (25), 4897-4910.
Martin, D. P., Willment, J. A., Billharz, R., Velders, R., Odhiambo, B., Njuguna, J., et al. ( 2001). Sequence Diversity and Virulence in Zea mays of Maize Streak Virus. Academic Press, 247-255
Martin, D. P. , & Shepherd, D. N. (September 2009). The epidemiology, economic impact and idwest Laboratoties. (2004). Soil Sampling. Omaha, NE: Midwest Laboratories, INC.
Ministry of Agriculture. (2014). Soil Suitability Evaluation For Maize Production in Kenya (pp. 194-200). Nairobi: National Accelerated Agricultural Inputs Access Program.
Mumtaz , M. Z., Aslam, M., Jamil , M., & Ahmad, M. (2014). Effect of Different Phosphorus Levels on Growth and Yield of Wheat under Water Stress Conditions. Journal of Environment and Earth Science, 4(19), 23-30.
Mwai G. N., J.C. Onyango and M.O.A. Onyango. 2001. Growth Responces of Spiderplant (Cleome gynandra L.) to Salinity. MSc. Thesis. Maseno University. p. 42.
NAFIS, (2016). Field Management. NAFIS. Retrieved 6 April 2016, from http://www.nafis.go.ke/agriculture/maize/field-management-practices/
Ngwira, P. & Khonje, P., (2005). Managing Maize Diseases through Breeding under Malawi Field Conditions. African Crop Science Conference Proceedings, Vol. 6. 340-345, 340-345.
Noellemeyer, E., & Six, J. (2015). Basic Principles of Soil Carbon Management for Multiple Ecosystem Benefits. Soil Carbon: Science, Management and Policy for Multiple Benefits, 265–276. https://doi.org/10.1079/9781780645322.0265
Obura P.A. (2008). Effects of soil properties on biodiversity of aluminium and Phosphorus in selected Kenyan and Brazilian soils. PhD Thesis, Purdue University, USA.
Olsen S.R., (1972). Micronutrient interactions in micronutrients in Agriculture. Eds J.J., Mortvedt. Soil Sci. Soc. Am., Inc., Madison, Wis. pp 243–264.
Okalebo, J. R., Gathua, K. W., & Woomer , P. L. (2002). Laboratory Methods of Soil and Plant Analysis: A Working Manual, Second edition. Nairobi, Kenya: SACRED Africa.
Onasanya R.R, Aiyelar O. P, Onasanya A, Oikeh S, Nwilene F.E and Oyelakin O.O. (2009). Growth and Yield Response of Maize (Zea mays L.) to Different Rates of Nitrogen and Phosphorus Fertilizers in Southern Nigeria. World Journal of Agricultural Sciences, 5 (4): 400-407.
Ouma E, Ligeyo D, Matonyei T, Agalo J, Were B, Too E, Onkware A, Gudu S, Kisinyo P and Nyangweso P. (2013). Enhancing Maize Grain Yield in Acid Soils of Western Kenya Using Aluminium Tolerant Germplasm. Journal of Agricultural Science and Technology , 33-46 .
Salehi, B., & Aminpanah, H. (2015). Effects of phosphorus fertilizer rate and Pseudomonas fluorescens strain on field pea (Pisum sativum subsp. arvense (L.) Asch.) growth and yield. Acta agriculturae Slovenica, 105(2), 213-214.
Satyaprakash, M., Nikitha, T., Reddi, E., Sadhana, B., & Vani, S. (2017). Phosphorus and Phosphate Solubilising Bacteria and their Role in Plant Nutrition. International Journal Of Current Microbiology And Applied Sciences, 6(4), 2133-2144. http://dx.doi.org/10.20546/ijcmas.2017.604.251
Scott M.P., Peterson J.M.,Hallauer A.R. (2009). Evaluation of combining ability of quality protein maize. Maydica, 54, 449-456.
Sharma, S., Sayyed, R., Trivedi, M., & Gobi, T. (2013). Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus, 2(1), 587. http://dx.doi.org/10.1186/2193-1801-2-587
Simões C.C, MeloJ.O, Magalhaes J.V and Guimarães C.T. (2012). Genetic and molecular mechanisms of Aluminium tolerance in plants. Genetics and Molecular Research, 1949- 1957.
Sun, Q., Shen, R., Zhao, X., Chen, R., & Dong, X. (2008). Phosphorus Enhances Al Resistance in Al-resistant Lespedeza bicolor but not in Al-sensitive L. cuneata Under Relatively High Al Stress. Annals Of Botany, 102(5), 795-804.
Too E.J, Were B.J, Onkware O.A, Ringo J.H, Carlsson A.S, Ouma E, Geleta M, Gudu S. (2014). Responce of selected Sorghum (Sorghum bicolor L.) to Aluminum stress. African Journal of Agricultural Research, 1651-1622.
Umeri, C., Moseri, H., & Onyemekonwu, R. (2016). Effects of Nitrogen and Phosphorus on the Growth Performance of Maize ( Zea mays ) in Selected Soils of Delta State, Nigeria. Advances in Crop Science and Technology, 4(1), 207-300.
Ward, C., Kleinert, A., Scortecci, K., Benedito, V., & Valentine, A. (2011). Phosphorus- deficiency reduces aluminium toxicity by altering uptake and metabolism of root zone carbon dioxide. Journal Of Plant Physiology, 168(5), 459-465.
Ware, G., Whitacre, D., Gunther, F., Albert, L., Voogt, P., Gerba, C., Hutzinger, O., Knaak, J., Mayer, F., Morgan, D., Park, D., Tjeerdema, R. and Yang, R. (2007) Reviews Of Environmental Contamination And Toxicology. Springer New York, New York, NY.
Yang J.C., Jiang H.M., Zhang J.F., Li L.L., Li G.H. (2013). Selection and Evaluation of Maize Genotypes Tolerance to Low Phosphorus Soils. IAEA TECDOC SERIES:TECDOC NO.1721 (pp. 55-77). Vienna (Austria): International Atomic Energy Agency, Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Soil and Water Management and Crop Nutrition Section.
Yousuf M, Saleem M (2001). Correlation analysis of S1 families of maize (Zea mays L.) for grain yield and its components. International Journal of Agricultural Biology. 3:387- 388.

